| Associate Editor, Stuart Sherman, MD, FASGE, recommends the article “Fully covered self-expandable metal stents for the treatment of benign biliary strictures” by Arthur J. Kaffes, MBBS, FRACP and Ken Liu, MBBS, BSci (Med) from the July issue of GIE. |
| This review article highlights the use of fully covered self-expandable metal stents (FCSEMSs) as a potential paradigm shift in our management of benign biliary strictures. This review is well-written and is encyclopedic in summarizing the world’s literature on the performance, clinical utility, and safety of FCSEMSs for the treatment of benign biliary strictures. As an evolving area of investigation, this is a very timely review. |
|
|
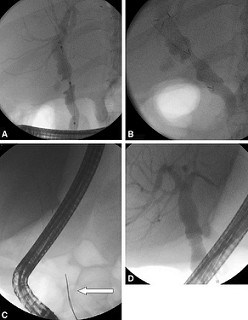
Figure 3. These fluoroscopic images illustrate one of the newer intraductal fully covered self-expandable metal stent type used in a 53-year-old woman with an anastomotic stricture after orthotopic liver transplantation (OLT). A, Cholangiogram clearly demonstrating a tight anastomotic stricture post-OLT. B, The FCSEMS (Niti-S Kaffes; Taewoong) is inserted across the stricture and is entirely within the biliary tree. C, The stent is seen in situ with the radiopaque removal string seen clearly in the duodenum (white arrow). D, The stricture has completely resolved. |
There are limited data on the use of FCSEMS and only 1 small RCT published as an abstract. Based on these limited data, it would appear that:
Read this article on pages 13-21 in the print journal or find it online. |
|