PULSTA™ Transcatheter Pulmonary Valve System



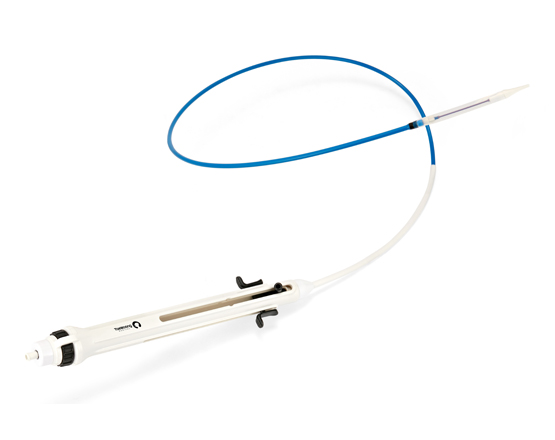

PULSTA™ Transcatheter Pulmonary Valve System.
KFDA approved (MFDS)
Video clip
Indication
For patients who require pulmonary valve replacement due to pulmonary valve regurgitation and/or stenosis
Features
● Self-expandable valve with Knitted-wire backbone
- no need to do pre-stent and applicable for native right ventricular outflow tract
● Various valve sizes up to 32mm
● Multi-step tissue engineering for longer durability
● Easy access and deployment at the main pulmonary artery landing zone
- low profile delivery system (18, 20 French)
- rather short loading length
● Simple and Easy Valve loading procedure
- no need to do pre-stent and applicable for native right ventricular outflow tract
● Various valve sizes up to 32mm
● Multi-step tissue engineering for longer durability
● Easy access and deployment at the main pulmonary artery landing zone
- low profile delivery system (18, 20 French)
- rather short loading length
● Simple and Easy Valve loading procedure
Articles
● Novel self-expandable, stent-based transcatheter pulmonic valve : a preclinical animla study.
Kim GB, Lim HG, Kim YJ, Choi EY, Kwon BS, Jeong S [Int J Cardiol. 2014 Apr 15;173(1):74-9.]
● First in human experience of a new self-expandable percutaneous pulmonary valve implantation using knitted nitinol-wire and tri-leaflet porcine pericardial valve in the native right ventricular outflow tract.
Catheter Cardiovasc Interv. 2017 Apr;89(5):906-909 Kim GB
● Successful Feasibility Human Trial of a New-Self-Expandable Percutaneous Pulmonary Valve (Pulsta™ Valve) Implantation Using Knitted Nitinol Wire Backbone and Trileaflet α-Gal-Free Porcin Pericardial Valve in the Native Right Ventricular Outflow Tract.
Circ Cardiovasc Interv. 2018 Jun; 11(6): e006494 Kim GB
● Early Outcomes of Percutaneous Pulmonary Valve Implantation with Pulsta and Melody valves : the First Report from Korea
Choi JY, Kim AY, Jung JW, SHing JI, EUN YM, Kim NK. [J. Clini. Med. 2020, 9, 2769; doi:10.3390/jcm909269]
● Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract
Lee S-Y, Kim GB, Kim S-H, Jang S-I, Choi JY, Kang IS, et al. [Catheter Cardiovasc Interv. 2021; 1-9, https://doi.org/10.1002/ccd.29865]
● Bilateral branch pulmonary artery Pulsta valve implantation for treatment of large right ventricular outflow tract in a high-risk patient
KIM JY, KIM S-H, Jang SI [Catheter Cardiovasc Interv. 2021;1-5. https://doi.org/10.1002.ccd.29857]
● Early results of Pulsta transcatheter heart valve in patients with enlarged right ventricular outflow tract and severe pulmonary regurgitation due to transannular patch
Odemis E, Yenidogan I, and Kizilkaya MH [Cardiology in the Young, 2022, page 1 of 9, doi: 10.1017/S1047951122003511]
● Outcomes of Transcatheter Pulmonary Valve Replacement and Surgical Pulmonary Valve Replacement : A Cohort Analysis
Kritvikrom Durongpisitkul et al. [Journal of the Society for Cardiovascular Angiography & Interventions, 2022, DOI:https://doi.org/10.1016/j.jscai.2022.100408]
● Self-expanding Pulmonary Valves in Fifty-Three Patients with Native Repaired Right Ventricular Outflow Tracts
Jou-Kou Wang et al. [Canadian Journal of Cardiology (2023), doi: https://doi.org/10.1016/j.cjca.2023.03.013]
Kim GB, Lim HG, Kim YJ, Choi EY, Kwon BS, Jeong S [Int J Cardiol. 2014 Apr 15;173(1):74-9.]
● First in human experience of a new self-expandable percutaneous pulmonary valve implantation using knitted nitinol-wire and tri-leaflet porcine pericardial valve in the native right ventricular outflow tract.
Catheter Cardiovasc Interv. 2017 Apr;89(5):906-909 Kim GB
● Successful Feasibility Human Trial of a New-Self-Expandable Percutaneous Pulmonary Valve (Pulsta™ Valve) Implantation Using Knitted Nitinol Wire Backbone and Trileaflet α-Gal-Free Porcin Pericardial Valve in the Native Right Ventricular Outflow Tract.
Circ Cardiovasc Interv. 2018 Jun; 11(6): e006494 Kim GB
● Early Outcomes of Percutaneous Pulmonary Valve Implantation with Pulsta and Melody valves : the First Report from Korea
Choi JY, Kim AY, Jung JW, SHing JI, EUN YM, Kim NK. [J. Clini. Med. 2020, 9, 2769; doi:10.3390/jcm909269]
● Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract
Lee S-Y, Kim GB, Kim S-H, Jang S-I, Choi JY, Kang IS, et al. [Catheter Cardiovasc Interv. 2021; 1-9, https://doi.org/10.1002/ccd.29865]
● Bilateral branch pulmonary artery Pulsta valve implantation for treatment of large right ventricular outflow tract in a high-risk patient
KIM JY, KIM S-H, Jang SI [Catheter Cardiovasc Interv. 2021;1-5. https://doi.org/10.1002.ccd.29857]
● Early results of Pulsta transcatheter heart valve in patients with enlarged right ventricular outflow tract and severe pulmonary regurgitation due to transannular patch
Odemis E, Yenidogan I, and Kizilkaya MH [Cardiology in the Young, 2022, page 1 of 9, doi: 10.1017/S1047951122003511]
● Outcomes of Transcatheter Pulmonary Valve Replacement and Surgical Pulmonary Valve Replacement : A Cohort Analysis
Kritvikrom Durongpisitkul et al. [Journal of the Society for Cardiovascular Angiography & Interventions, 2022, DOI:https://doi.org/10.1016/j.jscai.2022.100408]
● Self-expanding Pulmonary Valves in Fifty-Three Patients with Native Repaired Right Ventricular Outflow Tracts
Jou-Kou Wang et al. [Canadian Journal of Cardiology (2023), doi: https://doi.org/10.1016/j.cjca.2023.03.013]
