-
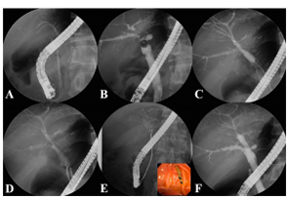 Salvage therapy using self-expandable metal stents for recalcitrant anastomotic strictures after liv
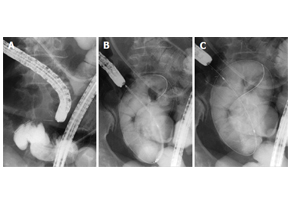
Salvage therapy using self-expandable metal stents for recalcitrant anastomotic strictures after livTherap Adv Gastroenterol. 2017 Mar; 10(3): 297–309. Salvage therapy using self-expandable metal stents for recalcitrant anastomotic strictures after living-donor liver transplantation Consequently, the aim of this study was to examine the feasibility and efficacy of using a newly designed fully covered self-expandable metal stent (FCSEMS) to resolve refractory ABS. A total of 35 FCSEMSs were positioned endoscopically and removed after 2-3 months. The anastomotic stricture resolved with the FCSEMS insertion in 29 of 35 patients (clinical success rate: 82.9%). The newly designed FCSEMS: KAFFES™ Stent is a potentially feasible and effective treatment for anastomotic strictures that develop after LT but are not amenable to treatment by conventional procedures. Insertion procedure for multiple FCSEMSs. (A) Previously inserted plastic stents are removed endoscopically. (B) The cholangiogram shows multiple anastomotic strictures at the posterior and inferior intrahepatic ducts. (C) The strictures are dilated using a balloon dilator to allow passage of the FCSEMSs. (D) The FCSEMSs are inserted sequentially into the stricture sites. (E) After an indwelling time of 2–3 months, the FCSEMSs are removed by grasping the retrieval strings using biopsy forceps. (F) The cholangiogram demonstrates resolution of the multiple strictures.
17.04.10 -
 Hands-on training of ENDO 2017 (Hyderabad, India)
Hands-on training of ENDO 2017 (Hyderabad, India)Hands-on training of ENDO 2017 (Hyderabad, India) Taewoong Medical supported a hands-on training program by KSGE (Korea Society of Gastrointestinal Endoscopy) and TAGE (Thai Association of Gastrointestinal Endoscopy) at the ENDO 2017 (16th-19th Feb) conference in Hyderabad, India. KSGE Hands-on Training (Topic: Metal stenting)KSGE hands-on training provided to give the over 40 participants chance to actually insert and deploy the plastic and/or metal stent endoscopically in the dummy model. This training program consisted of 6 stations including esophagus and/or pylorus stenting, colon stenting, bile duct plastic stenting, CBD metal stenting and hilar bile duct stenting (stent-in-stent and stent-by-stent). Honorable ERCP and/or Endoscopy masters, KSGE members offered one-on-one coaching and shared their valuable clinical skills and knowledge. The participants could have enhanced their skills on basic cannulation, stricture management with self-expandable metal stents. TAGE Hands-on Training (Topic: EUS-guided drainage)At this program, expert tutoring was available at one station for fine needle aspiration cytology and biopsy, two stations for EUS biliary drainage with metal stent and three stations for EUS pseudocyst drainage with plastic stent and NAGI™ and SPAXUS™ stent. Participants had a proctoring opportunity to observe and experience advanced therapeutic EUS techniques.
17.03.02 -
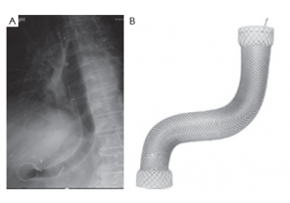 A novel management of post-oesophagectomy gastro-pleural fistula
A novel management of post-oesophagectomy gastro-pleural fistulaJ Gastrointest Oncol 2016;7(6):E93-E97 A novel management of post-oesophagectomy gastro-pleural fistula Javaid Ishtiaq1, Jonathan Sutton2, Waqar Ahmed2 AbstractOesophageal anastomotic leak and fistula are major and life-threatening complications of oesophagectomy with resultant increased mortality. Non-operative approach of such cases should be the initial strategy. Re-operative surgery and/or stent insertion are considered if conservative measures failed. Although oesophageal stenting is a safe option for the leaks, stent migration and failure to completely cover large anastomotic leaks are the main complications and pitfalls of the procedure. These can be overcome by using multiple or larger stents. We describe a case of a 73-year-old man who underwent a laparoscopic oesophagectomy for an oesophageal adenocarcinoma. The procedure was complicated by a large gastro-pleural fistula and anastomotic leak, resulting into a chronic empyema. The initial conservative treatment and a conventional oesophageal stent insertion failed to heal the fistula and to resolve the empyema. Re-operative surgery was ruled out because of the patient’s poor general health and high surgical risk. Due to the changed oesophago-gastric anatomy and a potential risk of migration of the additional conventional stent, a mega stent was deployed with successful closure of the oesophageal leak. Post-stenting contrast studies and an out-patient follow up review of the case confirmed no further anastomotic leakage.KeywordsGastrointestinal fistula; oesophageal leak; oesophageal stent; oesophageal cancer; mega stent Figure 3. The large (Mega) stent covering the oesophagus, stomach and pylorus with its proximal end in the previously deployed oesophageal stent and the distal end in the duodenum (A). The Mega stent (Taewoong Medical Niti-S Gyeonggi-do, South Korea oesophageal covered stent) (B). Correspondence to: Dr. Javaid Ishtiaq. Specialty Registrar, Gastroenterology, Sandwell and West Birmingham Hospitals NHS Trust, Lyndon, West Bromwich, West Midlands, B71 4HJ, UK. Email: javaidishtiaq@yahoo.com.
17.02.28 -
 2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program)
2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program)2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program) 2nd AERAT (Asan Medical Center EUS-guided Radiofrequency Ablation Training program) was held in Seoul, Korea from 6th to 7th January. Like last 1st AERAT training, many doctors participated in this time. A total of 12 doctors from HongKong, Malaysia, Singapore, India and Japan attedned and experienced various EUS related programs for 1night 2days. AERAT’s director Prof. Dong Wan, Seo gave a lecture related with EUS-procedure cases and his valuable experiences with attendees. Also, we prepared hands on session at Asan Meical Center Animal Laboratory with Phatom model. ex-vivo and procine model tests as well. All of attendees had their own experiences with our EUSRA probe. All the participants gave a good feedback that AERAT program was a satisfactory training course. We hope to meet many doctors in our RFA traiing class near in the future.
17.01.20 -
 Niti-S™ Colon D Stent_malignant small bowel obstruction
Niti-S™ Colon D Stent_malignant small bowel obstructionWorld J Gastroenterol. 2016 Oct 28; 22(40): 9022–9027. Safety and efficacy of self-expandable metallic stents in malignant small bowel obstructions Akiyoshi Tsuboi, Toshio Kuwai, Tomoyuki Nishimura, Sumio Iio, Takeshi Mori, Hiroki Imagawa, Toshiki Yamaguchi, Atsushi Yamaguchi, Hirotaka Kouno, Hiroshi Kohno AbstractIn this report, we present 3 cases of malignant small bowel obstruction, treated with palliative care using endoscopic self-expandable metallic stent (SEMS) placement, with the aim to identify the safety and efficacy of this procedure. Baseline patient characteristics, procedure methods, procedure time, technical and clinical success rates, complications, and patient outcomes were obtained. All 3 patients had pancreatic cancer with small bowel strictures. One patient received the SEMS using colonoscopy, while the other 2 patients received SEMS placement via double balloon endoscopy using the through-the-overtube technique. The median procedure time was 104 min. The technical and clinical success rates were 100%. Post-treatment, obstructive symptoms in all patients improved, and a low-residue diet could be tolerated. All stents remained within the patients until their deaths. The median overall survival time (stent patency time) was 76 d. SEMS placement is safe and effective as a palliative treatment for malignant small bowel obstruction.KeywordsSelf-expandable metallic stents; Malignant small bowel obstructions; Endoscopy; Case report; Pancreatic cancer Figure 1 Self-expandable metallic stent deployment using the standard through-the-scope technique under fluoroscopic guidance. A: The scope was advanced to the stricture, and a standard guidewire was passed through the stricture; B: The stent delivery system was advanced through the scope across the stricture; C: The stent can be seen successfully deployed across the stricture.
17.01.04 -
 Niti-S™ NAGI™ Stent_A two-center Comparative study of plastic and FCSEMS stent in PFCs
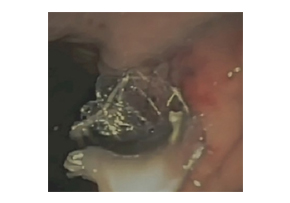
Niti-S™ NAGI™ Stent_A two-center Comparative study of plastic and FCSEMS stent in PFCsEndosc Ultrasound. 2016 Sep-Oct; 5(5): 320–327. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections Tiing Leong Ang, Pradermchai Kongkam,1 Andrew Boon Eu Kwek, Piyachai Orkoonsawat,1 Rungsun Rerknimitr,1 and Kwong Ming Fock Background and ObjectivesEndoscopic ultrasound-guided drainage of walled-off pancreatic fluid collections (PFCs) (pseudocyst [PC]; walled-off necrosis [WON]) utilizes double pigtail plastic stents (PS) and the newer large diameter fully covered self-expandable stents (FCSEMS) customized for PFC drainage. This study examined the impact of type of stent on clinical outcomes and costs.Patients and MethodsRetrospective two-center study. Outcome variables were technical and clinical success, need for repeat procedures, need for direct endoscopic necrosectomy (DEN), and procedure-related costs.ResultsA total of 49 (PC: 31, WON: 18) patients were analyzed. Initially, PS was used in 37 and FCSEMS in 12. Repeat transmural drainage was required in 14 (PS: 13 [9 treated with PS, 4 treated with FCSEMS]; FCSEMS: 1 [treated with PS]) due to stent migration (PS: 3; FCSEMS: 1) or inadequate drainage (PS: 10). Technical success was 100%. Initial clinical success was 64.9% (25/38) for PS versus 91.7% (11/12) for FCSEMS (P = 0.074). With repeat transmural stenting, final clinical success was achieved in 94.6% and 100%, respectively (P = 0.411). Compared to FCSEMS, PS was associated with greater need for repeat drainage (34.2% vs. 6.3%, P = 0.032). The need for and frequency of DEN was similar between both groups, but PS required more frequent balloon dilatation. PS was significantly cheaper for noninfected PC. Costs were similar for infected PC and WON.ConclusionPS was associated with a higher need for a second drainage procedure to achieve clinical success. The use of FCSEMS did not increase procedural costs for infected PC and WON.KeywordsEndosonography, endotherapy, necrosectomy, pancreatic necrosis, pseudocyst Figure 3. Endoscopic image of Nagi™ stent inserted for drainage of infected walled-off necrosis
17.01.03 -
 EUS-guided RFA Master Course Program (Seoul, Marseille)
EUS-guided RFA Master Course Program (Seoul, Marseille)EUS-guided procedures are no longer the future of EUS but rather the present! Nowadays EUS has emerged as an essential field in diagnostic and therapeutic endoscopy. Our EUS-guided RFA master course is not only to provide the opportunities to understand mechanism of EUS-guided RFA, but also to learn the essential technical tips for successful RFA applications through comprehensive lectures and hands-on courses including patient’s case observation and animal test, phantom hands-on. We are convinced that our comprehensive and intensive 2 days course will guide all of attendees to EUS-guided RFA program practice as well as introduce our EUS-RFA novel device. We are completed twice of EUS-guided RFA master course successfully until now in Seoul, Korea and Marseille, France.Let’s share the program inside story together from now.EUS-guided RFA Master Course Program is primarily focused on the following training courses and lectures. First program was held in Seoul, Korea Asan Medical Center directed by Prof. Seo, Dong Wan and second program was held in Marseille, France Nord Hospital directed by Prof. Marc Barthet. Both training program were consisted of EUS lectures by director and EUS-RFA case observation, and hands-on with phantom model and porcine model. EUS lectures are open to questions from the participant’s review of case observation and hands-on training in a preliminary session.This one-to-one interaction training program will hopefully build up links and long term relationships helpful for personal development and a greatest integration of future procedure.Most of the participants hope that EUSRA™ can be applied to a variety of indication not only pancreas tumor but also, gradually predict increase these EUS treatment in the future.
16.12.05 -
 Taewoong Medical Research Center Open
Taewoong Medical Research Center OpenTaewoong Medical Research Center has newly-organized a preclinical team to provide preclinical evaluation service for medical devices ‘Build a foundation for systematic research to support R&D and provide histological analysis.’ Taewoong Medical has been continuously investing in product development since its foundation in 1999 and engaged in organic research cooperation with many research institutions at home and abroad, making efforts to diversify and improve the functionality of the product. This year in March, a preclinical team has been established in Taewoong Medical’s Osong research center.Preclinical Studies are executed to test a medical device in animals before conducting any clinical trials in the patient to verify biological safety and effectiveness of the medical device. Biocompatibility testing and risk assessment for marketing approval of medical devices necessarily entails accurate analysis of data from biological evaluation and animal testing. Therefore, the newly-organized preclinical team is attracting expectations to enhance the competitiveness of the product while improving the safety in medical procedures.The new preclinical team provides expert research support services to various medical personnel and researchers, not to mention supporting Taewoong’s own products in development. The team has already been entrusted with animal tissue with biomaterial implant from several universities and research institutions and has embarked on the histological analysis service.For those researchers who require animal experiments will benefit from a laboratory animal center of Osong Medical device development center (Department of evaluation Bio Compatibility assessment team; www.kiobhealth.kr). A researcher-centered ‘One-way system’ embracing from the whole process of animal testing to histological analysis will be introduced in the near future. In addition, to aid the safe and correct use of commercial products, the domestic and international sales divisions are preparing to implement training programs using experimental animals targeted for medical specialists.With the installation of the new preclinical team, Taewoong Medical can internally evaluate cell testing and animal testing from early stages of development to the point of completing a new product, which will enable Taewoong to introduce mature products to the market in a much shorter period of time. Also, offering targeted training programs to medical specialists and histological analysis service will enhance efficiency and research achievements of medical devices.
16.12.05 -
 Niti-S™ SPAXUS™_Preliminary report_EUS-Guided Drainage of Pseudocyst_Korea
Niti-S™ SPAXUS™_Preliminary report_EUS-Guided Drainage of Pseudocyst_KoreaPreliminary report on a EUS guided pseudocyst drainage with a new lumen apposing metal stent (SPAXUS™); single center, 9 months experience Background and AimsEndoscopic ultrasound (EUS)-guided drainage has become a mainstay for treating peripancreatic fluid collection and fully covered metal stent provided more efficient drainage than plastic stent. Recently, wide-flanged fully covered metal stent is introduced in EUS-guided pseudocyst drainage and showed promising results. The aim of this study was to evaluate the feasibility and efficacy of EUS-guided pseudocyst drainage with a new lumen apposing metal stent (Niti-S™ SPAXUS™ Stent; Taewoong Medical, Seoul, Korea).MethodA prospective, single-arm, multicenter feasibility study of EUS-guide pseudocyst drainage with SPAXUS™ is now enrolling patients at 6 tertiary hospital in Korea. This preliminary report describes single center, 9 months experiences of the multicenter study. Between January and September 2016, a total of 11 patients were treated with EUS-guided pseudocyst drainage with SPAXUS™ in our institute. Baseline characteristics and outcomes including technical/clinical success rate, adverse event, and recurrence rate were evaluated.ResultThe median age of 11 patients was 57 (42-76) and male was 10 of 11 patients. The median size of PFC was 80 mm (60-160 mm). SPAXUS™ was successfully placed all patients and PFC resolution was achieved in all 11 patients at median of 35 days after stent placement. The median procedure time was 7 min (5-10 min). Three cases of fever were developed during immediate post-procedure period and all of them were successfully treated with intravenous antibiotics. No other immediate post-procedure adverse events were seen. One case of stent occlusion was developed 40 days after procedure and endoscopic stent cleaning with retrieval balloon was conducted. Until now, no case of PFC recurrence was observed.ConclusionEUS-guided pseudocyst drainage with SPAXUS™ was conducted effectively and safely in our institute until now. Further multicenter experience would be helpful to clarify the role of this procedure.-----
16.12.05 -
 CONNECT GLOBAL issue 7, OCT 2016 16.10.07
CONNECT GLOBAL issue 7, OCT 2016 16.10.07 -
 IDEN 2016 (International Digestive Endoscopy Network) Seoul, Korea
IDEN 2016 (International Digestive Endoscopy Network) Seoul, KoreaIDEN 2016 (International Digestive Endoscopy Network) Seoul, Korea International Digestive Endoscopy Network (IDEN) 2016 was held in Seoul, Korea from June 24 to June 26, 2016. At IDEN congress, many physicians and researchers from all over the world would participated to present and discuss the latest advances in the field of upper GI, lower GI and pancreatobiliary endoscopy. During IDEN congress period, we invited key opinion leaders and our partners to the Taewoong Medical factory to introduce our production process. Stent production tour was arranged to look all around the each production rooms, in addition we had Endoscopic RFA hands-on experience.“It was first time to see how to make hand-made metal stent and impressive the many steps for producing the one stent.” said one of participants.We also prepared Korean traditional dinner and had a great time with all of them.We have invited KOLs and partners to Korea twice an annual event on June and October during IDEN and SGI domestic congresses. Upcoming next official factory tour will be held on October 6 before the day of SGI congress. To learn more about SGI and factory tour, please contact your territory manager.
16.07.05 -
 Niti-S™ SPAXUS™ Stent won the excellent patent award
Niti-S™ SPAXUS™ Stent won the excellent patent award[2016 상반기 우수특허 대상]태웅메디칼‘자가 팽창 스텐트’, 두 장기를 연결한국일보 : [2016 상반기 우수특허 대상] 태웅메디칼태웅메디칼(대표 신경민, www.stent.net)은 1991년 설립된 의료기기 제조 및 수출기업이다. 태웅메디칼은 약 60여개 국가에 연간 3,000만달러 이상의 수출을 하고 있으며 2014년 미국지사를 설립해 적극적인 해외투자에 나서고 있다. 특히 세계적으로 품질의 우수성을 인정받고 있는 스텐트(인체내강 확장용 의료기기)의 지속적인 제품 개발을 위해 많은 투자를 하고 있다.태웅메디칼이 이번에 개발한 ‘Niti-S SPAXUS 스텐트’는 형상기억합금인 나이티놀 와이어를 사용해 제작된 자가 팽창형 금속 스텐트이다. 두 장기를 연결할 수 있도록 스텐트의 양쪽 끝 단에 두 장기를 연결하고 고정할 수 있는 기술이 적용됐다.이 스텐트의 특징은 형상기억합금 와이어를 교차되게 엮어 제작한 몸통 부위와 날개 부위로 이뤄져 있으며 스텐트는 실리콘 피막으로 코팅돼 있다. 스텐트의 날개부위는 인접된 두 장기 사이에 밀착해 연결할 수 있으며, 몸통부위는 내경을 확보해 배수 및 이동경로 변경의 통로를 만들어준다.태웅메디칼은 소화기내과용 스텐트에 머무르지 않고 비뇨기과용, 뇌혈관, 약물방출 등의 스텐트를 지속적으로 개발할 계획이다.http://hankookilbo.com/v/e665b273a5c945efb86984caacdc9bcf
16.06.10 -
 DDW 2016 (Digestive Disease Week), San Diego, USA
DDW 2016 (Digestive Disease Week), San Diego, USADDW 2016 (Digestive Disease Week), San Diego, USA Taewoong Medical participated DDW 2016 with a huge attention of attendees in San Diego, USA. Exhibition booth consisted of SPAXUS™ Stent section, Endoscopic RFA area, Stent area and extended meeting rooms. Design concept was simple and modern, which emphasized full exposure of our company identity. Especially we prepared to demonstrate SPAXUS™ user friendly delivery system so that visitors could try easy deployment. The day before DDW, Taewoong Medical organized partner’s day with global distributor at Holiday Inn, Bayside. We had SPAXUS Hands on class with special dummy model and a lively discussion time about EUSRA & ELRA, Endoscopic RFA products. In particular, Euromedical, Italy presented their sales strategy and current state of RFA business, and this invaluable sharing would stand partners in good stead when it comes to meeting customers. Mr. Shin, CEO, Taewoong Medical, expressed thanks to the all partners regarding their effort and cooperation in closing remark. 2nd day of DDW congress, the valued customers and friends from all of the world were invited to SeaWorld, attraction of San Diego. Taewoong Medical prepared special show with killer whales and dinner buffet beside the pool. All attendees seem to return to the innocence of childhood during the show, and they had a great time even though some of them were dripping wet by whales.Upcoming next DDW 2017 is going to be held at Chicago. Hope we can get together again!
16.06.07 -
 Website Renewal to become more easily to access even if you can access mobile device
Website Renewal to become more easily to access even if you can access mobile deviceWebsite Renewal to become more easily to access even if you can access mobile device Have you visited our website lately?You can see optimize pages whichever way you look at it. PC Screen Shot We have finished website renewal to become more easily to access and use even if you can access mobile device and also renewed the entire design and the structure of previous website in order to make easier to use and more convenience for obtaining necessary information with regard to our business activities and products for valuable customers and business partners through brand new website. We aim to proactively and effectively deliver updated valuable information regarding our business activities, products and services to customers and we also utilize our website as a part of sales and marketing tools for global users. As you know we have also opened Facebook webpage and we always aim to have a better communication with partners and customers! Please visit the updated TaeWoong Website and send us the sincere feedback! Mobile Screen Shot Through recent update, following new pages and products been added into the website.Customer Service: You can find General product information which is frequently asked like MRI Information, Storage.Meet Us At: Find congress and TW event informationProduct:M Biliary StentEndoscopic Accessory OPTIMOS™
16.04.01 -
 Niti-S™ NAGI™ Stent_Author Discussion Series : Natsuyo Yamamoto
Niti-S™ NAGI™ Stent_Author Discussion Series : Natsuyo YamamotoNatsuyo Yamamoto, MD, PhD, from The University of Tokyo writes about her article“Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections.” Although efficacy of endoscopic cystogastrostomy for pancreatic fluid collections is reported, few stents dedicated for this purpose are commercially available. We evaluated a newly developed, short metal stent with wide lumen diameter customized for cystogastrostomy in 9 cases. Technical success rate was 100% without early adverse events. Direct endoscopic necrosectomy through the stent was successful in all 3 attempted cases. Stent removal was achieved without difficulties in all 6 cases. THE NEW FULLY COVERED METAL STENT (NAGI STENT, TAEWOONG-MEDICAL CO, LTD, YEONGGI-DO, KOREA) The placement of this metal stent in the treatment of pancreatic fluid collection may prevent complications such as migration and peritonitis. A wide stent lumen enables direct endoscopic necrosectomy through the stent.A plastic stent has been commonly used for enterocystostomy. Endoscopic treatment for pancreatic fluid collection including necrosectomy is technically feasible using this new metal stent. The safety, efficacy, and indication should be evaluated in larger prospective studies.Read the abstract for this article here.The information presented in Endoscopedia reflects the opinions of the authors and does not represent the position of the American Society for Gastrointestinal Endoscopy (ASGE). ASGE expressly disclaims any warranties or guarantees, expressed or implied, and is not liable for damages of any kind in connection with the material, information, or procedures set forth.
16.03.19 -
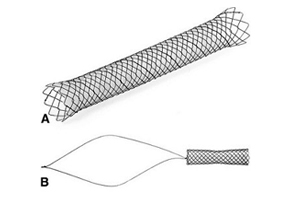 Niti-S™ KAFFES™ Stent_ Author Discussion Series: Ken Liu
Niti-S™ KAFFES™ Stent_ Author Discussion Series: Ken LiuKen Liu, Faculty of Medicine at the University of Sydney in Camperdown, New South Wales, Australia, reports on his Review Article“Fully covered self-expandable metal stents for treatment of benign biliary strictures.” The focus of this paper was the use of fully covered self-expandable metal stents (FCSEMS) in the treatment of benign biliary strictures (BBSs). There has been an explosion of recent research on the use of FCSEMSs in BBSs with suggestions of a paradigm shift from using plastic stents. Due to their growing popularity, we felt it was important to review the available literature on the efficacy, safety, and cost-effectiveness of FCSEMSs in BBSs.FCSEMSs have been shown to achieve excellent success rates in both transplant and non-transplant BBSs (>90% in some studies). This success is sustained for over 12 months in most studies. The efficacy of FCSEMSs is unchanged when used as first-line or as second-line therapy (after balloon dilation and plastic stenting). The subset of patients with BBSs secondary to chronic pancreatitis remains a challenge (46-75% success). Results of FCSEMSs in BBSs are similar to studies of plastic stents, in particular, studies using multiple plastic stents. Fewer ERCP sessions are required for treatment of BBSs with FCSEMSs which may result in cost savings, although published data are lacking. Figure 1. A, An example of the classic stent type (Bonastent; Standard Sci-Tech). B, An example of a new stent type (Niti-S Kaffes; Taewoong). The stent sits entirely within the biliary tree with a long removal string that rests in the duodenum. The antimigration feature in this stent is the gradual tapering in the middle of the stent FCSEMSs are safe to use. Adverse events such as pancreatitis, cholangitis, secondary strictures, and pain are infrequent and usually respond to conservative management. Spontaneous stent migration rates are variable; however, several anti-migration modifications have demonstrated efficacy in reducing this adverse event.There is still a need for further studies, in particular, randomized controlled trials comparing FCSEMSs vs. plastic stents. Data are also lacking regarding optimal duration of stent placement, and the cost effectiveness and long term safety of FCSEMSs.Read this article on pages 13-21 of the print Journal or find it online.The information presented in Endoscopedia reflects the opinions of the authors and does not represent the position of the American Society for Gastrointestinal Endoscopy (ASGE). ASGE expressly disclaims any warranties or guarantees, expressed or implied, and is not liable for damages of any kind in connection with the material, information, or procedures set forth.
16.03.19
+82 31-904-6196contact@stent.net
HOME
NEWS
News
